Tap into Excelya’s late phase and real world evidence (RWE) experience for all you peri-approval and post-marketing clinical study needs.
Late phase studies and real-world data (RWD) give you the real-world evidence (RWE) you need to help change patients’ lives and improve outcomes.
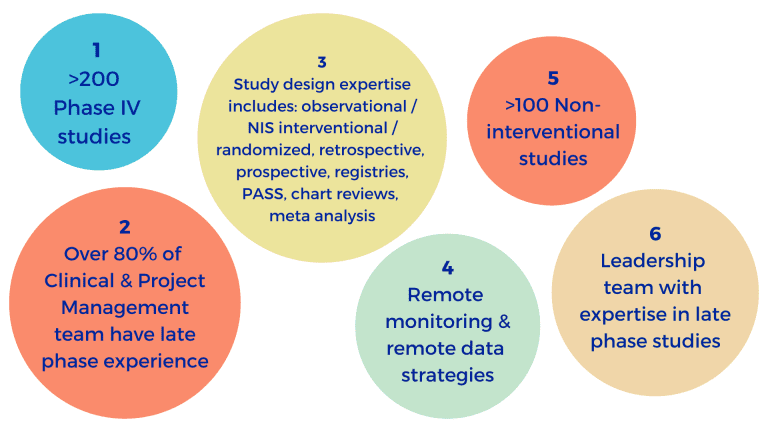
At Excelya, we have conducted more than 200 late phase studies including over 100 non-interventional studies (NIS) across multiple regions. You can apply our knowledge to your trial from the very beginning – we have a subject matter expert for outcome research study designs, as well as expertise across different study designs:
When you work with our talent pool of 900+ experts, you tap into our deep understanding of everything from peri-approval and post-marketing study design to operational best practices. Even if you lack experience and expertise at trial sites, our experienced late-phase specialist CRAs can give you the support you need at budget you can work with.


Our experience enhances your clinical trial, resulting in the best possible outcomes for your research and those who benefit from it; Excelya’s leadership team has NIS and RWE experience and more than 80% of our Clinical and Project Management team have experience with late-phase clinical trials.
Our late phase and RWE experience spans therapeutic areas and geographies, giving you insights you can use to accelerate your journey to market. We also have access to specialist experts across a range of therapeutic areas.
Excelya’s experts have worked on:
• Over 200 phase IV studies and over 100 non-interventional studies
• Various study designs, including observational, interventional, randomized, retrospective, registry and meta analyses
• Remote monitoring and remote data strategies
Whatever your late phase trial looks like, we have the people who can help you succeed.
The challenges involved in late phase clinical trials and RWE, particularly around regulation and monitoring, can delay or even halt your trial.
We understand what makes RWE and late phase trials different, and we know how to overcome the obstacles you might face. Our CRAs have extensive experience in regulatory processes across different countries, and we have a subject matter expert on the regulatory framework in Europe for different peri-approval and post-marketing study designs.
We can help you navigate the regulatory pathways and operational requirements, helping you generate data you can use to help patients.

Observational or non-interventional studies (NIS) can provide valuable RWE, helping you determine the long-term safety and performance of an intervention.
But they also come with challenges. Observational studies tend to be very long and require remote monitoring, for example.
We know that understanding the patient journey in the real world is key to study success. At Excelya, we take a flexible approach to remote monitoring that can evolve as your observational trial progresses.
You can benefit from our expertise through every stage of your NIS. By putting people first, we ensure all your stakeholders’ needs are met.
When you run a late-phase or RWE trial, there can be many stakeholders to take into account, including patients, providers, payers and regulators. Communication with sites is key to your trial’s success, which is why all our CRAs are site relationship managers.
Our specialist CRAs all have high-caliber phase IV experience, and expertise on the nuances of phase IV and NIS study approaches. They have worked with numerous sites and they know how to communicate with them effectively.
With proven remote monitoring strategies and specialist NIS processes, our CRAs are equipped to manage any situation, helping you reach your goals.

Real world data can be highly valuable, but collecting it brings challenges: you may have to deal with large volumes of data from a variety of sources in RWE, such as devices, wearables and eCOA.
At Excelya, we know the questions that unearth optimal data to help you reach your goals. Our experts can help optimize your clinical trial for data, from study design through monitoring to statistical analysis.
Our data experts use various tools and questionnaires, including ePRO, eCOA, right fit EDC systems, questionnaires and QoLs, and they have expertise in data structures, unstructured data and analysis. We also use technology for a lighter data collection approach that can last the duration of your trial.